Dissociate Protoplast Protocol
Introduction

Materials
Biological materials
Young plant.
Reagents
- MES (Sigma, cat. no. M8250)
- Mannitol (Sigma, cat.no.M4125)
- CaCl2 (Sigma, cat.no. C7902)
- KCl (Sigma, cat.no. P3911)
- MgCl2 (Sigma, cat.no. M9272)
- NaCl (BBI, cat.no.7647-14-5)
- CellulaseR10 (YakultPharmaceuticalInd. Co., Ltd., Japan)
- MacerozymeR10 (YakultPharmaceuticalInd. Co., Ltd., Japan)
- Bovine Serum Albumin (BSA) (Cat. No. #A8020, Solarbio, Beijing, China)
- HemiCellulase (Sigma)
Equipment
- Cell Strainer (40 Micrometre, cat.no.F613461-9001)
- Scalpel
- Petri dish
- 10mL or 50mL centrifuge tube and 2mL centrifuge tube
- Centrifuge with adjustable acceleration
Reagent setup
Enzymolysis Solution
| Component | Amount (10ml) | |
|---|---|---|
| CellulaseR10 | 0.15g | |
| HemiCellulase | 0.1g | |
| MacerozymeR10 | 0.075g | |
| Mannitol | 5ml | |
| KCl | 0.2ml | |
| MES | 2ml | |
Water bath at 55 degree celsius for 10 minutes, then add ddH2O (0.2ml), CaCl2 (100 Micrometre) and BSA (0.01g) in sequence.
Procedure
Preparation of protoplast suspension
1. Enzymatic hydrolysis of tissue samples
Plant tissue were cut into 1-2 mm cuttings. Pour the previously prepared enzymolysis solution into the petri dish, add the cut hypocotyls to the enzymolysis solution (ensure that the tissue is completely immersed in the enzymolysis solution), shake at room temperature at a low speed, and avoid light for 4 -6 hours.
2. Filtration suspension
The single cell suspension after enzymolysis was filtered with a 40 Micrometre Cell Strainer. Take the filtered cell suspension into a centrifuge tube, add twice the volume of PBS (with 0.04% BSA) and mix it in an ultra-low speed centrifuge, centrifuge at 70g for 3 minutes, discard the supernatant, repeat this step, and finally leave a certain amount of precipitate, Mix well with a cut pipette tip, filter into a new centrifuge tube with a 40 Micrometre cell strainer.
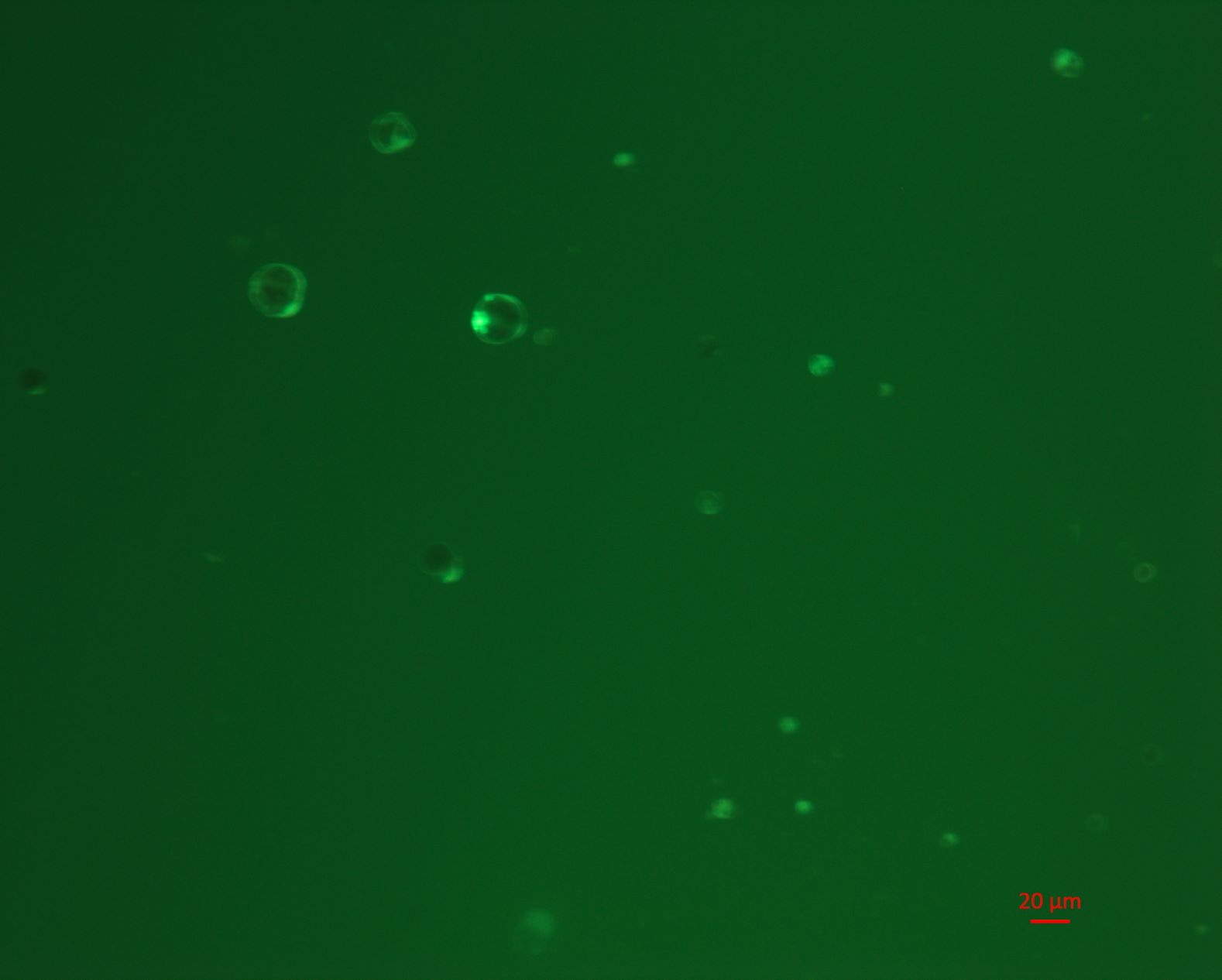
3. Microscopy for cell state
A small amount of suspension was dripped onto the slide and observe the cell number and cell viability (tested by Fluorescein Diacetate) under a microscope.
4. Reverse transcription and 10X genomics library construction
Approximately 15,000 counted cells were used for reverse transcription and subsequently library preparation according to instruction.
Troubleshooting
| Step | Problem | Solution |
|---|---|---|
| 2 | Low cell concentration in suspension | The enzymolysis time needs to be determined according to the actual situation. Generally, the enzymolysis can be completed by seeing a certain number of protoplasts under the microscope. |
Anticipated results